Molecular Biology in context
In biology, structure determines function across scales. We are a multidisciplinary team of scientists who use biochemistry, structural biology, cell biology and bioinformatics to study the structures underlying key cellular processes within their native cellular environment. Where the right tools are unavailable, we build our own. We apply methods from materials science, physics, signal processing and computer vision to build new tools to visualize the molecular details of the cell.
Structural cell biology
Methods for determining or predicting the structures of isolated proteins are well established. We now have large “structureomic” databases that serve as a reference for the scientific community. But understanding how a single protein’s structure contributes to its role in cell functions like growth, division and response to viruses, which involve interconnected networks of proteins acting in the context of higher order structures in the cell, is a major challenge. We aim to develop methods to place molecular structures in their cellular context.
RNA structure in context
Like protein, RNA adopts 3D structures that can affect its function. Unlike most proteins, RNA typically has more than one likely structure and exists as a conformational ensemble. The composition of this ensemble can be altered by the cellular and sequence context. We aim to understand the cellular factors that constrain RNA structures. How does the structure of the nucleolus affect the folding of rRNA and ribosome biogenesis? How do transcription, nuclear export and translation affect the structure of mRNA 3’UTRs?
Current Projects
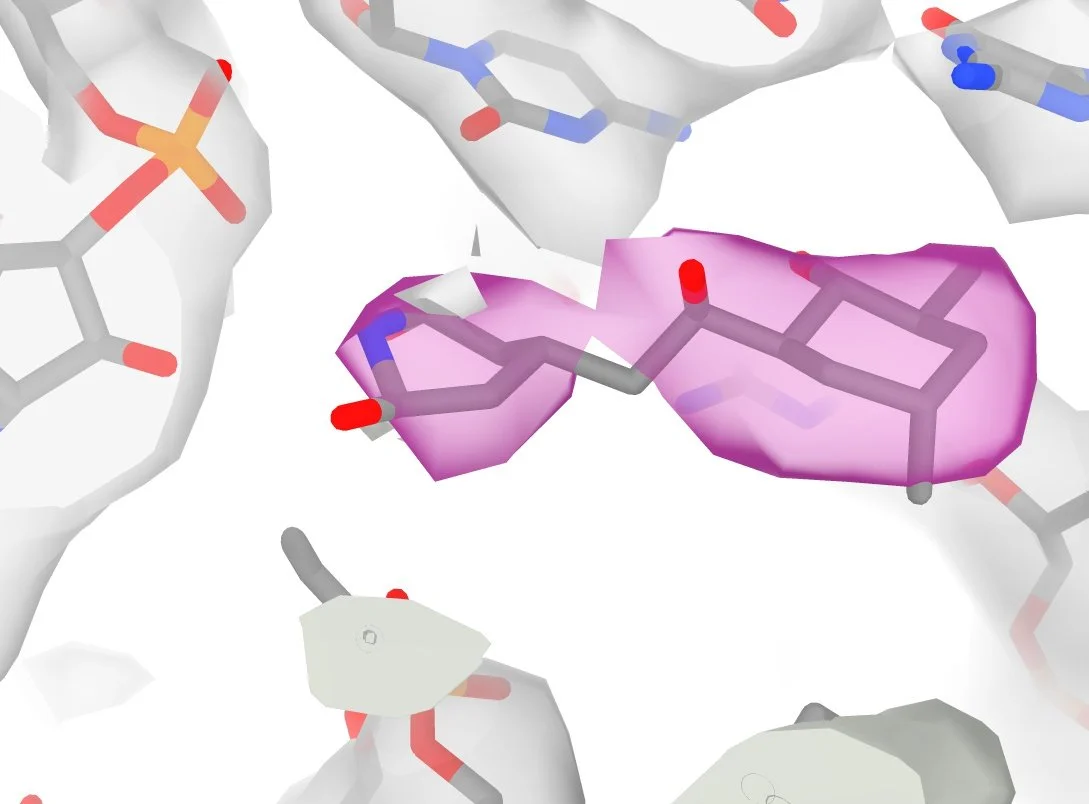
Cryo-EM tools for structural cell biology
We use focused ion beam (FIB-milling) and cryogenic electron microscopy (cryo-EM) to image macromolecules in thin sections of frozen cells. Identifying structures within these images is a major challenge for two reasons: 1) because the density of molecules in the cell makes it difficult to separate overlapping features and 2) we only have partial information for each molecule. Together, this makes cryo-EM images of cells extremely low contrast and low SNR.
We get around this by using 2D-template matching (2DTM): a strategy which allows us to compare the image with the expected signal predicted from a known structure. (more on 2DTM here and here). We previously showed that we could use this to classify related structures in cells, assign probabilities to single molecules and generate high-resolution reconstructions. We are developing new strategies for template matching to increase speed and sensitivity as well as methods that use 2DTM to read out structure from 2D images of cells.
Improved methods for cryo FIB-milling
Building structural models of cellular pathways requires single molecule precision. Focused ion beam (FIB)-milling is used to generate thin sections of cryogenically frozen cells to image with cryo-EM. However, typical protocols introduce damage, limiting our ability to image single molecules in cells. We are testing alternate strategies to generate thin lamellae with minimal damage without slowing down the process.
mRNA structures regulating localization, translation and turnover
Transcription of the genetic code from DNA to RNA is the first step in gene expression. Messenger (m)RNAs carry the genetic code that is translated by the ribosome to protein. mRNAs also contain untranslated regions (UTRs) at their 5' and 3' ends that can extend to thousands of nucleotides, in some cases, many times longer than the translated region. RNA structure in UTRs can regulate mRNA localization, translation and turnover, and therefore affect gene expression. Identifying mRNA UTR structural elements that affect mRNA localization, translation and turnover and the rules that govern RNA folding are crucial to understand gene expression and to inform the design of mRNA therapeutics.
Visualizing ribosome biogenesis in the nucleolus
Ribosomes are the modular molecular machines that are responsible for faithfully translating the genetic code to produce every cellular protein. Ribosome assembly is the most energy demanding process in a growing cell, consuming more than half of available energy. In yeast >200 ribosome biogenesis factor proteins are required to assemble the 4 rRNAs and ~80 proteins to produce ~2,000 ribosomes per minute. Structures of purified intermediates in the assembly pathway have revealed how some of these 200 biogenesis factors aid assembly. However, in cells, ribosome assembly spans three subcellular compartments, the nucleolus, the nucleus and the cytoplasm. How the detailed molecular arrangements are coordinated in the cell is unclear. We make use of new cryo-EM methods to visualize intermediates of ribosome assembly from the nucleolus to the cytoplasm. Using this approach, we can correlated the detailed molecular structures of intermediates with their subcellular location at high resolution. In this way, we aim to build a spatially resolved molecular model of an entire biological pathway.